Cleverusername5321
Member
- Joined
- Oct 22, 2013
- Messages
- 142
- Gender
- Male
- HSC
- N/A
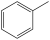
My teacher told us that toluene is not an alkene because it's not actually double bonds that occur in it. We tested this by adding bromine water to toluene, which did not discolour it, which would seem to suggest that it indeed does not have double bonds. My teacher also showed our class an image of the bonds that actually occur, but I forgot the name. Anyone know the name of these bonds?