Thanks for that contribution, you got me thinking that can't be right because IUPAC has reserved the term "sol" for colloidal suspensions, so I went to the references.
In the Journal of Pure Appl. Chem., Vol. 80, No. 2, pp. 233–276, 2008. doi:10.1351/pac200880020233 © 2008 IUPAC they give the approved references for all the Physical Chemistry terms, and this paper recommends the term "sln" for state of solution.
Thanks for that information.
I can't say that I am surprised that IUPAC decided to declare a new practice that differs from one already in use. It doesn't mean that many will follow it, however. It can see the sense in not using a single term (sol) with different meanings, but that doesn't mean the recommendation will be followed - and it is one that I don't recall having heard.
In any case, whether using "(sol)" or "(sln)", the important point for the HSC is that it is inappropriate to use the "(l)" state for other than pure liquids or a solvent. It leads to confusion.
For example, if I dissolve sodium chloride into water, I write:
NaCl (s) -----> Na+ (aq) + Cl- (aq)
If I heat and melt solid sodium chloride, I write:
NaCl (s) -----> Na+ (l) + Cl- (l)
If I dissolve sodium chloride into liquid ammonia, writing any of
NaCl (s) -----> Na+ (sol) + Cl- (sol)
or
NaCl (s) -----> Na+ (sln) + Cl- (sln)
or
NaCl (s) -----> Na+(in NH3, l) + Cl-(in NH3, l)
is preferable to the highly misleading option of
NaCl (s) -----> Na+ (l) + Cl- (l)
which looks like melting. I might clarify a little by putting something over the arrow, like:
NaCl (s) --H2O--> Na+ (aq) + Cl- (aq)
NaCl (s) --heat--> Na+ (l) + Cl- (l)
NaCl (s) --NH3--> Na+ (l) + Cl- (l)
but the opportunities for confusion remain high.
It also makes confusing a case like Fischer esterification where the solvent is the mixture of components, albeit with generally the higher concentration of the alcohol, because a system like
CH3COOH (sln) + CH3CH2OH (sln) <--- ---> CH3COOCH2CH3 (sln) + H2O (sln)
having an equilibrium constant of
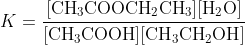
is a lot more intuitively obvious for an HSC student than making the same claim about an equilibrium system written as
CH3COOH (l) + CH3CH2OH (l) <--- ---> CH3COOCH2CH3 (l) + H2O (l)
I hope you agree.


