Hello Everyone, I was just wondering if the identification of anion and cation will be needed for the HSC Chemistry exam, as in does it come up often in the exam and is it worth studying? If so what are the anions and cations that we need to know?
Cation and Anion Identification? (1 Viewer)
- Thread starter Newbit
- Start date
DefiningTheta
Member
- Joined
- Aug 6, 2012
- Messages
- 124
- Gender
- Male
- HSC
- 2012
- Uni Grad
- 2018
don't forget the flame tests as well.
For Iron do you need to know the test for both Fe2+ and Fe3+?Yeah its in the syllabus so it can definitely come up. You need to know how to test for:
phosphate, sulfate, carbonate, chloride, barium, calcium, lead, copper, iron
Yeah you doFor Iron do you need to know the test for both Fe2+ and Fe3+?
Fe2+ >>>>>> White ppt with OH- but quickly turns brown
Fe3+>>>>>> Blood red ppt with SCN-
someth1ng
Retired Nov '14
This is correct.Yeah its in the syllabus so it can definitely come up. You need to know how to test for:
phosphate, sulfate, carbonate, chloride, barium, calcium, lead, copper, iron
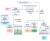
There's many different ways to do it but the way I do it is the HSCOnline method.
For anions:
1. Add Fe3+ (brown ppt of OH-)
2. Add Fe2+ (ppt is of CO3 2- or PO4 3+ --> Add acid and carbonates will produce effervescence of CO2)
3. Add Ba2+ (white ppt of SO4 2-)
4. Add Pb2+ (white ppt of Cl-)
For cations:
1. Add Cl- (white ppt for Pb2+, grey ppt that darkens for Ag+)
2. Add SO4 2- (white ppt for Ca2+ and Ba2+ --> flame test)
3. Add OH- (ppt of Cu2+ and Fe2+ and Fe3+ --> Add NH3 in copper and a blue solution is produced, Fe2+ is green but turns brown, Fe3+ is only brown)