This is a little out of syllabus, but it will help you understand BCS theory I think. What are known as electron-cooper pairs are formed because as the electrons move through the lattice, the positive charges shift slighty towards the path of the electrons since positives are attracted to it. This creates a net small positive charge in the middle of the electrons path on their way to the positive terminal.
Now, this small positive charge which is significant, attracts more and more electrons, so on the one hand the electrons are trying to stay away from each other, on the other they want to get to the mini positive charge.
If the conditions are suitable, a quantum mechanical effect happens, and the electrons form cooper pairs.
The reason why cooper pairs are useful, are because while electrons are known as thermions , cooper pairs are known as bozons. And the Pauli exclusion principle which states that no 2 thermions can occupy the same energy state. So cooper pairs are bozons and hence are not effected by Pauli Exclusion principle, which effectively allows a lot of cooper pairs to be formed and they can essentially pass through each other (someone please correct me if I'm wrong).
The reason why such a low temperature is needed to form cooper pairs, is because the amount of energy needed to form a cooper pair is 10^{-3} eV (around that), and if that energy is overcome, then the cooper pair will split.
(out of syllabus):
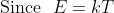
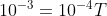
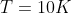
k is Boltzman's constant. So it can be proven that temperature needs to be around 10 Kelvin in order for cooper pairs to be formed in general.
If I explained something wrong or it is incorrect, someone please tell me.

