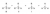Honestly, I simply hate phrasing it as "tertiary alcohols are more reactive than primary alcohols". Why? It is completely reaction-dependent and absolutely incorrect to answer this question without referring to the
exact reaction you are looking at. For example, tertiary alcohols are far less reactive to oxidation reactions. I'm going to assume this is specifically the
reaction of an alcohol with a hydrogen halide acid (i.e. an SN1 reaction).
As for r1ckworthy's answer, IMO, total bullshit. It is inaccurate to refer to any polarization or weakening of the C-O bond as a cause for the increased rate of the SN1 reaction. The increased substitution just...doesn't really do that much for the
neutral molecule. The rate of a reaction is dependent on the
activation energy (Eact1), which involves the reactant and the transition state only. Also, the reasoning was total nonsense (e.g. "positive and negative repel"? Seriously?).
There are several steps in this reaction:
- Protonation, where the alcohol gets protonated by the acid (protonations are usually fast and reversible).
- Ionisation, where water is released to generate the carbocation (generally the rate-determining step)
- The carbocation is captured by the halogen ion (usually fast and technically reversible to some extent).

The potential energy diagram will look something like this (in this case, X = H2O, Nu = halogen), showing steps two and three only:

In this diagram, the first step has a large energy barrier to generate the carbocation intermediate (in the middle of the saddle). To understand why tertiary alcohols are more reactive, you need to know
why the activation energy (i.e. Eact1) is smaller when using tertiary alcohols. For this, you need to know about
Hammond's postulate:
"If two states, as, for example, a transition state and an unstable intermediate, occur consecutively during a reaction process and have nearly the same energy content, their interconversion will involve only a small reorganization of the molecular structures."
That is, the energy of the carbocation and the adjacent transition state (i.e. the peak) is closer in energy (compared to the protonated alcohol and the transition state), so they will be more structurally similar. In other words,
the transition state will resemble the carbocation (or is carbocation-like). From here, we can talk about substitution and how it can stabilise a carbocation. The diagram below ranks most stable to least stable (i.e. more substitution = more stable carbocation).
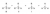
Now, to understand why it's more stable...it just comes down to how adjacent C-H or C-C bonds contain electrons and those bonds can partially overlap with the empty p-orbital of the carbocation (essentially donating some electron density to the carbocation, diagram below). So in fact, the more substituted carbocation is
less positively charged (at the carbon centre) than a less substituted carbocation. Remember what I said before: the transition state resembles the intermediate/carbocation (Hammond's postulate). Therefore, things that stabilise the carbocation will also stabilise the transition state (lowering both potential energies, second diagram), lowering the activation energy (Eact1) and increasing the reaction rate. This means for the tertiary carbocation, you are accumulating
less positive charge on any single atom.

Also, for the protonated alcohol, the potential energies are largely unaffected by substitution because substitution does not stabilise the positive charge on the oxygen. Therefore, I focused on the transition state and carbocation intermediate where substitution has a large impact. If you're curious, the second transition state (i.e. halide capture) is also carbocation-like, so increased substitution will stabilise that too.
tl;dr more substitution stabilises the carbocation intermediate, whose formation is the rate-determining step. adjacent C-H bonds can donate some electron density to the carbocation, which lowers its energy and helps its formation.