The relevant syllabus dot point here is
● analyse the contribution of Schrödinger to the current model of the atom
Now, this hasn't been tested in HSC yet so there's a high probability that this may be in this year's hsc. Also, it's very broad in what it wants. I don't have the most knowledge about it but here's what I know.
Basically, firstly know about the thought experiment analogy (Schrodinger's cat) which, to put it simply, if you have a cat in a box, you don't know if it's dead or alive until you actually check. It's actually can be both states at once and this is called superposition. These dead and alive states were analogies of quantum states of an electron which IDK if we need to know but the ones I know are spin and magnetic moment (?). But really these are just empty words if we don't know what they mean which makes me question why they included this dot point. Also, know about how Shrodinger found out about the energy equation of an electron
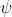
in the form of a 3d wave function which is imaginary so instead he uses
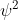
. So basically, his equations give an approximate model to the probability of the position of an electron in a 3d space, which we don't actually know of unless we check, most likely in an orbital of an atom. He also made the orbital model which looks like this.
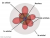
Chem kids would already know about this I think so yay another unfair advantage this subject gives to chemistry students.
Besides these, know about the Pauli exclusion principle (no fermions (quarks and leptons) can occupy simultaneously all 4 quantum states (principle number, spin, angular momentum, and magnetic moment)), and the Heisenberg uncertainty principle
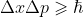
(more you know about momentum, less you know about the position and vice versa) which again is not that important unless you're doing the ib which actually uses this principle in questions.
NB: importantly, know that this model overcame limitations of the Bohr model, i.e. explained why everything was quantized among others like Zeeman.